Hugh Jackman’s Second Skin Cancer Scare
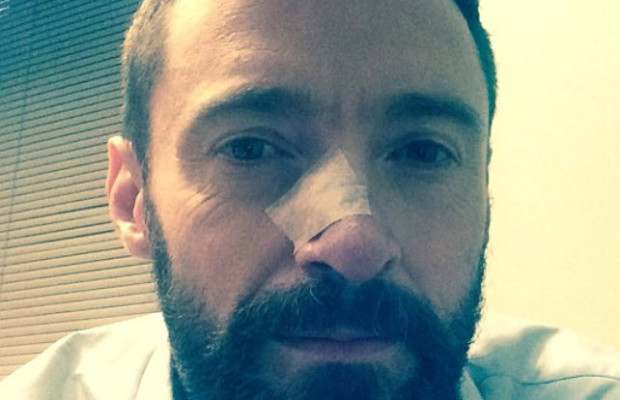
Hugh Jackman has again taken to Instagram to make an announcement. He’s had another basal cell cancer removed from his nose.
“Another Basel Cell Carsinoma (sic). All out now. Thanks Dr. Albom and Dr. Arian. PLEASE! PLEASE! WEAR SUNSCREEN!.”
It was just in November that Jackman revealed that he had first been diagnosed with skin cancer.
What you need to know about sunscreen!
Spending time in the sun increases the risk of skin cancer and early skin aging. To reduce this risk, consumers should regularly use sun protection measures including:
- Use Broad Spectrum sunscreens with SPF values of 15 or higher regularly and as directed
- Reapply sunscreen at least every 2 hours, more often if you’re sweating or jumping in and out of the water.
- Limit time in the sun, especially between the hours of 10 a.m. and 2 p.m., when the sun’s rays are most intense.
- Wear clothing to cover skin exposed to the sun; for example, long-sleeved shirts, pants, sunglasses, and broad-brimmed hats
In June 2011 FDA announced significant changes to sunscreen products that will help consumers decide how to buy and use sunscreen, and allow them to more effectively protect themselves and their families from sun-induced damage.
In a nut shell, the new regulations do the following:
- Broad Spectrum designation. Sunscreens that pass FDA’s broad spectrum test procedure, which measures a product’s UVA protection relative to its UVB protection, may be labeled as “Broad Spectrum SPF [value]” on the front label. Broad Spectrum SPF products with SPF values higher than 15 provide greater protection and may claim additional uses, as described below.
- Use claims. Only Broad Spectrum sunscreens with an SPF value of 15 or higher can claim to reduce the risk of skin cancer and early skin aging if used as directed with other sun protection measures. Non-Broad Spectrum sunscreens and Broad Spectrum sunscreens with an SPF value between 2 and 14 can only claim to help prevent sunburn.
- “Waterproof, “sweatproof” or “sunblock” claims. Manufacturers cannot label sunscreens as “waterproof” or “sweatproof,” or identify their products as “sunblocks,” because these claims overstate their effectiveness. No sunscreen is completely waterproof, sweatproof or can totally block the sun’s rays! Sunscreens cannot claim to provide sun protection for more than 2 hours without reapplication or to provide protection immediately after application (for example– “instant protection”) without submitting data to support these claims and obtaining FDA approval.
- Water resistance claims. Water resistance claims on the front label must indicate whether the sunscreen remains effective for 40 minutes or 80 minutes while swimming or sweating, based on standard testing. Sunscreens that are not water resistant must include a direction instructing consumers to use a water resistant sunscreen if swimming or sweating.
- Drug Facts. All sunscreens must include standard “Drug Facts” information on the back and/or side of the container. This is similar to the labels found on most other over-the-counter medications. It includes a list of the active and inactive ingredients, warnings and directions for use.
For more information, go to the FDA website at www.fda.gov/sunscreen.


























0 comments