GOOP Under Fire

The watchdog group Truth In Advertising (Tina) is urging two California District Attorneys to investigate Gwyneth Paltrow‘s lifestyle and health website, GOOP, for “unsubstantiated, and therefore deceptive, health and disease-treatment claims.”
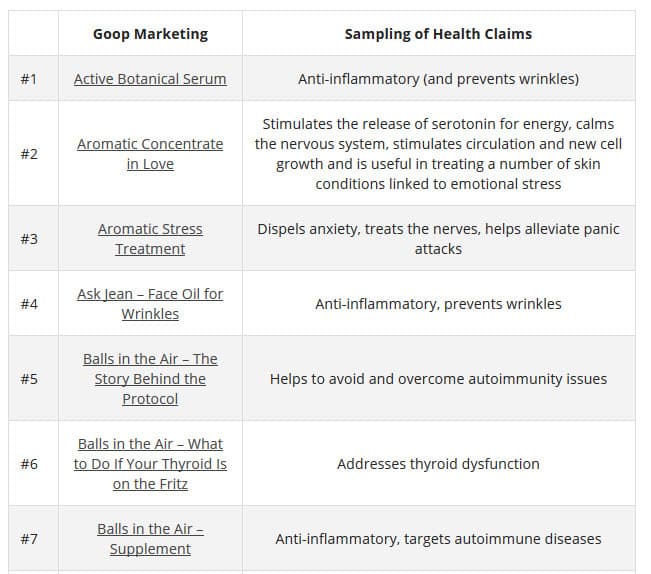
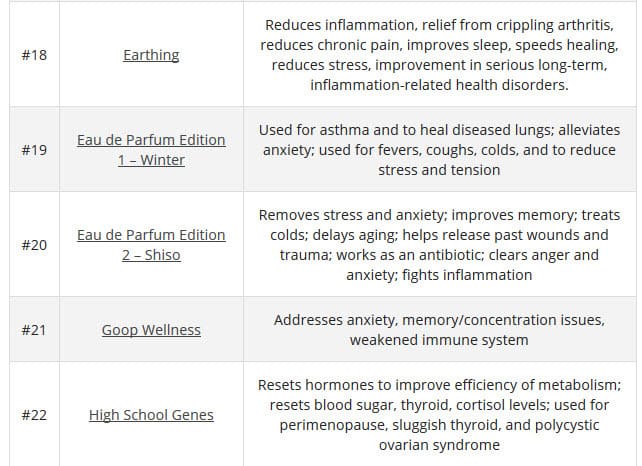
According to TINA.org, their investigation ” into Goop’s marketing has revealed more than 50 instances in which the company claims, either expressly or implicitly, that its products (or those it promotes) can treat, cure, prevent, alleviate the symptoms of, or reduce the risk of developing a number of ailments. These include crystal harmonics for infertility, rose flower essence tincture for depression, black rose bar for psoriasis, wearable stickers for anxiety, and vitamin D3 for cancer. The problem is that the company does not possess the competent and reliable scientific evidence required by law to make such claims.”
Here are a few of the claims on the GOOP website:
On August 11, Truth in Advertising sent letters to the company, its celebrity founder (Paltrow) and CEO. In the letter, TINA.org signaled its intent to alert government regulators if GOOP did not take corrective action by Aug. 18. In addition:
“TINA.org provided the company with a list of Goop and Goop-promoted webpages containing illegal health claims. Despite being handed this information, Goop to date has only made limited changes to its marketing.”
This prompted TINA to file their complaint with the CA District Attorneys.
The FDA (Food and Drug Administration) is the government agency responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, and cosmetics.
The Federal Food, Drug, and Cosmetic Act (FD&C), passed by Congress in 1938, gives authority to the FDA to oversee the safety of food, drugs, and cosmetics. It also gives them jurisdiction to monitor claims made in the labeling about both the composition and the health benefits of foods.
The FD&C:
- Bans false and misleading statements from the labeling of foods, drugs, medical devices, cosmetics, and tobacco.
- Defines the term “drug” to include, among other things, “articles intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease,” as well as “articles (other than food) intended to affect the structure or any function of the body.”
Health fraud scams refer to products that claim to prevent, treat, or cure diseases or other health conditions, but are not proven safe and effective for those uses. Health fraud scams waste money and can lead to delays in getting proper diagnosis and treatment. They can also cause serious or even fatal injuries.
The FDA has produced this video to help you recognize a potential health fraud scam:



























0 comments